কার্নো ইঞ্জিন ও কার্নো চক্র
An ideal refrigerator operates according to the reverse Carnot cycle and transmits heat from a cold source with water at a temperature of to a boiler with water at a temperature of . What amount of water must be frozen in the cooler to convert 1 kg of water into vapor in the boiler ?
Step 1:
Calculate the enthalpy of vaporization (ΔHv) at 27°C
ΔHv = 2256.6 kJ/kg
Step 2:
Calculate the amount of heat required to vaporize 1 kg of water
Q = ΔHv = 2256.6 kJ/kg
Step 3:
Calculate the amount of water that must be frozen in the cooler
Amount of water frozen = Q / (Tc - Tf)
Amount of water frozen = 2256.6 kJ/kg / (100°C - 27°C)
Amount of water frozen = 4.94 kg
Ai এর মাধ্যমে
১০ লক্ষ+ প্রশ্ন ডাটাবেজ
প্র্যাকটিস এর মাধ্যমে নিজেকে তৈরি করে ফেলো
উত্তর দিবে তোমার বই থেকে ও তোমার মত করে।
সারা দেশের শিক্ষার্থীদের মধ্যে নিজের অবস্থান যাচাই
কার্নো ইঞ্জিনের প্রতি স্তরে সংকোচন বা প্রসারণের অনুপাত 1: 6, এতে কার্যনির্বাহক বস্তু হিসাবে ও মোল দ্বি-পরমাণুক গ্যাস ব্যবহার করা হলো। ( = 1.4)
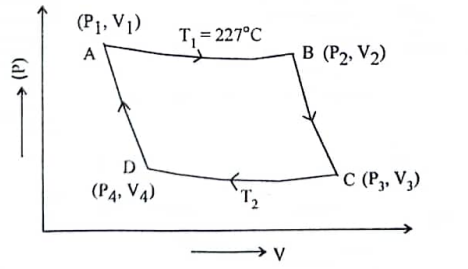
কোনো কার্নো ইঞ্জিনের দক্ষতা 75% এবং তাপ গ্রাহকের তাপমাত্রা 67°C । তাপ উৎসের তাপমাত্রা কত হবে?
কার্নোচক্রের চতুর্থ ধাপে নিচের কোনটি স্থির থাকে?
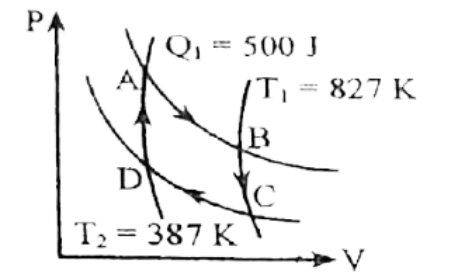 চিত্রে একটি কার্নো ইঞ্জিনের P-V লেখচিত্র দেখানো হল।
চিত্রে একটি কার্নো ইঞ্জিনের P-V লেখচিত্র দেখানো হল।